Investments in global health research and development (R&D) have led to the launches of many life-saving health technologies. Twenty years ago, for example, we had no malaria vaccine. Today, two effective malaria vaccines are being rolled out across Africa. And the future looks bright: The pipeline of candidate products for most global health conditions is more robust than ever (though some neglected diseases, such as leprosy, scabies, and trachoma, still have too few candidate products). In theory at least, the next 20 years could be even better than the last 20 years for launching breakthrough global health technologies.
However, strong headwinds are getting in the way of bringing new global health products quickly to market. The timelines for discovery, clinical trials, and regulatory approval can be lengthy, especially in low-income countries (LICs) and middle-income countries (MICs). Success rates are low: One study found that only around 1 in 20 drug development projects lead to a product launch. And late-stage clinical trials have become increasingly expensive (a phase 3 trial of a tuberculosis vaccine candidate is costing over $500 million), while annual funding for neglected disease R&D is falling.
Could innovations in the R&D ecosystem—new ways of discovering, testing, manufacturing, and financing health technologies—help overcome these headwinds and drive more efficient product development? In two new studies, we answer that question. Our research was based on conducting over 120 expert interviews, reviewing the literature, and applying quantitative modeling tools to estimate how innovations in the R&D ecosystem could affect the costs, success rate, and impact of future R&D efforts.
Six shifts in the R&D ecosystem could drive efficiencies
Our first study identified six ecosystem innovations that could make global health R&D faster and more efficient.
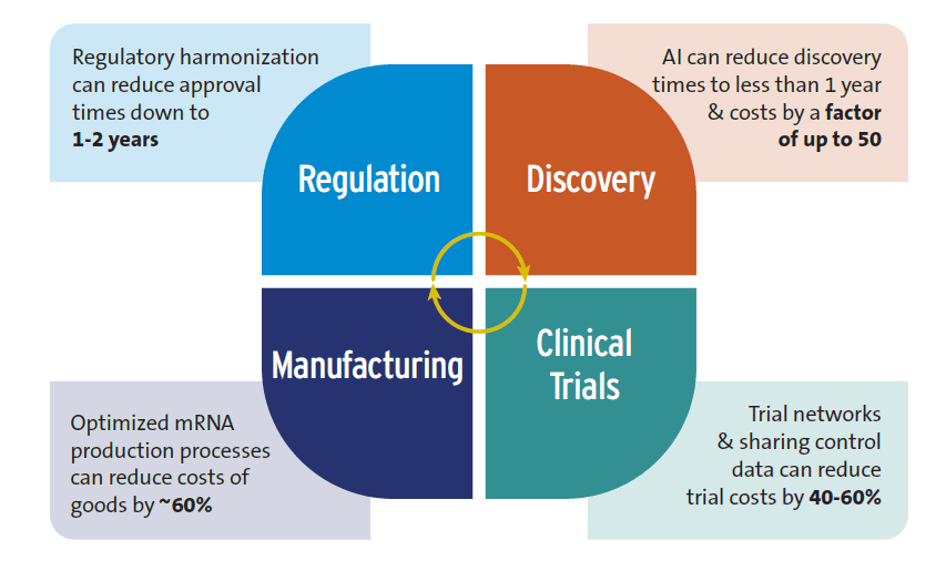
- Artificial Intelligence (AI). While there are some far-fetched claims—such as AI will replace human clinical trials—we found evidence that AI is already having a positive impact on global health R&D, especially on early-stage research. For example, the R&D discovery phase typically takes 3-5 years and costs up to $10 million. With AI, discovery can be shortened to less than 12 months, and there are AI tools that have lowered discovery costs by a factor of 50. Nevertheless, experts we interviewed cautioned that AI could worsen inequalities between high-income countries (HICs) and LICs and MICs if it is rolled out inequitably.
- Advances in trial design. A good example is clinical trial networks, which were critical to the development of both COVID-19 and mpox vaccines. These networks save time and money by using existing sites instead of creating new ones, recruiting patients more quickly, and reducing the number of patients needed by sharing control groups—costs could be reduced by 40-60% by sharing control groups and using control data from previous trials.
- New techniques for manufacturing drugs and vaccines. Modular manufacturing facilities have a much smaller footprint than traditional manufacturing plants, driving down capital costs. Using improved mRNA production approaches can also lower costs. If mRNA vaccine production processes are optimized, this could save over 60% of the annual cost of goods for the production of 100 million vaccine doses compared to conventional mRNA manufacturing. Such savings could lower mRNA vaccine production costs to just $0.5 per dose.
- Accelerating regulatory approval of new health technologies. There is a long delay in market authorization of new health products between HICs and LICs. One study found a lag of 4 to 7 years between first submission of new medicines for regulatory approval, which is usually to a regulator in a HIC, and final approval in sub-Saharan Africa. Three types of regulatory reform are reducing this time lag: regulatory harmonization (e.g., countries conducting joint regulatory reviews), strengthening national and regional regulatory capacity, and conducting rapid scientific reviews. Regulatory harmonization can reduce approval times down to 1-2 years.
- New types of health technologies, such as mRNA and monoclonal antibodies. As shown during COVID-19, mRNA platforms are versatile and suited for speed, which is especially valuable during pandemics. It will be critical for LICs and MICs to be able to manufacture their own mRNA technologies. However, the patent holders for many of the production inputs needed for mRNA are mostly in HICs, and overcoming this barrier will require a combination of stronger sharing of intellectual property and technology transfer agreements.
- Financing innovations. These are being used to tackle the constrained funding environment for global health R&D. One example is the U.S. priority review voucher (PRV) scheme—an incentive that rewards developers of a new health product for an eligible neglected disease with a tradeable voucher that grants priority review of a second product candidate (the voucher is worth around $100 million). The scheme has supported the development of new health tools for global health conditions. It may be beneficial for the European Union to explore the potential of implementing a similar PRV scheme.
Figure 1 summarizes how these ecosystem innovations could accelerate R&D and lower its costs.
Figure 1. Potential efficiency gains from shifts in the R&D ecosystem
Source: Authors’ research
Modeling the impacts of these efficiencies
In our second study, we developed quantitative models to estimate what would happen over a 22-year period, from 2023-2044, if some of the key innovations in the R&D ecosystem described above were to be widely adopted. One metric we used was societal net monetary benefits (NMBs), which we calculated as the difference between the economic value of the health benefits from a successful product launch and the incremental costs associated with developing, launching, and delivering the product to the target population.
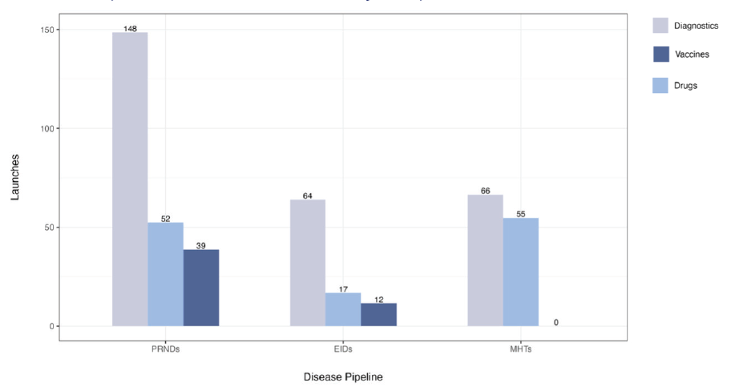
There are currently 1,498 candidate medicines, vaccines, and diagnostics in the product development pipeline for neglected diseases, emerging infectious diseases, and maternal health conditions. If none of the ecosystem innovations are adopted, and assuming there is no replenishment of this current pipeline, investing in R&D to advance the current candidates in the pipeline would yield an estimated 453 product launches from 2023 to 2044 (Figure 2). Of these 453 products, 42 would yield positive NMBs. However, 94 needed products would still be missing, e.g., new treatments and diagnostics for maternal iron deficiency anemia and fetal distress, and vaccines against six emerging infectious diseases (EIDs), including Crimean-Congo Hemorrhagic fever, Ebola, Marburg, Nipah, Rift Valley fever, and Zika.
How would this picture change if ecosystem innovations were adopted? Pooling and coordinating investments could lead to the launches of all 94 needed products, and the number of products yielding positive net monetary benefits to society would increase from 42 to 106. The incremental cost, over and above current annual R&D spending, would be an additional $1.4 billion to $7 billion annually (the range depends on the complexity of the product candidates being launched). If pooled and coordinated investments are combined with using AI and smarter clinical trial designs, we estimate there would be savings of over $9 billion and a reduction in the average cost per product launch by up to $100 million. The number of products yielding positive NMBs would increase to 110. Even greater efficiencies would be achieved if market entry was faster and production costs were lower; the number of products yielding positive NMBs would increase to 115.
If widely adopted, a range of innovations from AI to smarter product development approaches could make critical health tools available more quickly and at lower cost. Taken together, our two new reports suggest we could be on the cusp of a revolution in global health R&D.
Figure 2. Potential product launches from the current product development pipeline
Note: PRNDs: poverty-related and neglected diseases; EIDs: emerging infectious diseases; MHTs: maternal health technologies. Figure assumes no replenishment and no adoption of ecosystem innovations.








Commentary
Accelerating the discovery and development of new health technologies
July 22, 2024